Procope Medicals is moving resolutely into 2025 with new milestones and key challenges in the design and development of its total artificial heart. Here’s an overview of what we’ll be talking about:
Research and development (R&D)
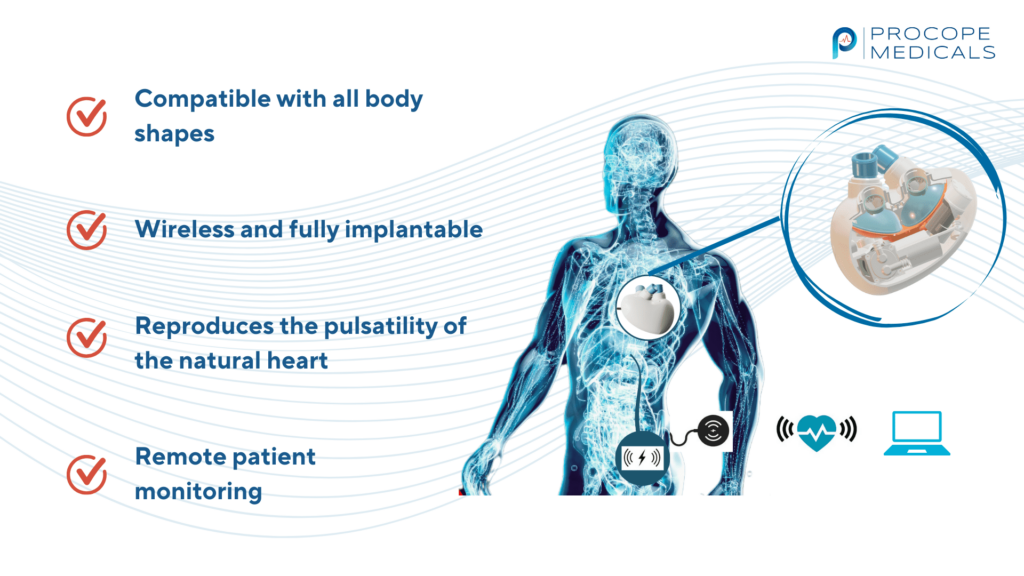
As we move into 2025, we will continue to develop the functional version of the prosthesis.
R&D actions over the next 12 months will enable us to develop the final technological building blocks required for the final version of the prosthesis:
- Development of the TET (recharging by energy induction)
- Development of control electronics
Intellectual property
Protecting our work is an essential aspect of our strategy. In 2025, we will be submitting two new technical advances in our artificial heart for patentability review. This is part of our drive to strengthen our position as a major player in heart health.


Financing
This strategic year includes a Series A with a fund-raising stage of 2 million euros, where we will be soliciting Venture Capital (VC) and Family Offices.
Structural actions
At Procope Medicals, we are reinforcing our commitment to quality and innovation, while ensuring that our procedures are streamlined.
Our priority is to focus on a strategic regulatory action plan, with the aim of obtaining CE marking, the certification guaranteeing that devices conform to rigorous standards.

Our multi-disciplinary team of engineers and physicians is determined to contribute to innovation in heart health and improve the quality of life of patients suffering from severe heart failure.
