Implantable medical devices are introduced into the human body through surgery or other medical interventions. Among the most common devices are artificial joints, breast implants, cochlear implants, intraocular lenses, pacemakers and other cardiac implants or prostheses.
How do these implantable devices work ?
Implantable medical devices are therefore placed in the human body on a permanent or temporary basis to support the functions of specific organs or tissues, monitor physiological activities or administer medication.
Some implants are prostheses designed to replace damaged body parts. They can be made from human tissue or exogenous materials such as metals, plastics and ceramics.
In France, 1,500 companies, 93% of them start-ups and SMEs, work in the medical device sector. This sector creates over 85,000 jobs and generates sales of around €31 billion. (French Ministry of the Economy, Finance and Industrial and Digital Sovereignty)
With an increasing prevalence of age-related chronic health conditions, the rapid growth of this industry has been observed worldwide. Certain emerging technologies, including the Cloud, the Internet of Things (IoT) and artificial intelligence, have contributed significantly to the advancement of the medical device industry.
Some of the leaders in the medical device industry: Medtronic (Ireland), Johnson & Johnson (USA), CardinalHealth (Ireland and USA), Abbott (Chicago), Siemens AG (Germany)…
The demanding path of implantable MD
Innovation in the medical device sector can be harder to achieve than in other sectors, the reason being specific regulations and requirements imposed on materials and companies, contributing to an explosion in development costs.
A new medical device must go through rigorous clinical studies to demonstrate its safety and efficacy before it can be CE-marked or approved by the FDA (USA). These studies involve the participation of surgeons and patients throughout the process, to assess the device’s performance under real-life conditions.
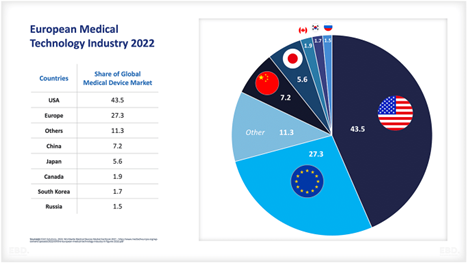
... But market dynamics are ever more present
Increasing demand for products and services in the field of medical device technologies, driven by factors such as population growth, urbanization and changing consumer preferences, is a major driver of market growth.
One of the most dynamic areas is active implantable medical devices, which are devices that are totally or partially inserted into the human body for diagnostic, monitoring or therapeutic purposes, and which are intended to remain in place.
Once on the market, these devices are continuously monitored to detect any patient safety issues. Manufacturers are required to report any incidents promptly to the competent authority.
Let’s not forget that the major objectives in the development of a new device are :
Its positive impact on patients: therapeutic success, ease of use, adherence to treatment.
Reducing the obstacles to innovation, i.e. cutting costs and speeding up time-to-market.
What are today's trends ?
The offer is highly varied in terms of the technologies on offer and the healthcare issues addressed. The market is growing at a rate of 5% a year.
To encourage this constant innovation, the French government has launched France 2030, a program designed to accelerate the transformation of this sector. Two major themes have been proposed, with the aim of creating world-class French leaders and developing innovative medical devices:
The first is “Robotics in surgery / Augmented surgery”, which aims to promote the use of new robots in surgery.
The second is “Implantable prostheses”, to highlight breakthrough innovations such as artificial hearts.

Procope Medicals has designed an implantable medical device in collaboration with cardiac surgeons at Nantes University Hospital. As part of our commitment to Health and Innovation, our Total Artificial Heart aims to significantly improve the quality of life of patients who have to live with this type of medical device. From 2024 onwards, Procope Medicals’ priority is to implement the strategic action plan with a view to obtaining CE marking by 2029, guaranteeing full compliance and transparency in our regulatory processes.
Sources :
https://www.economie.gouv.fr/dgccrf/Publications/Vie-pratique/Fiches-pratiques/Dispositifs-medicaux#:~:text=Lunettes%2C thermomètres%2C préservatifs%2C pansements,médicaux pour leur usage quotidien.
https://www.economie.gouv.fr/france-2030-developper-produire-dispositifs-medicaux#:~:text=Les chiffres clés du secteur,euros de chiffre d’affaires.
https://lne-gmed.com/fr/devices-of-focus/aimd
https://www.bpifrance.fr/nos-actualites/dispositifs-medicaux-un-secteur-en-croissance
Implants and Prosthetics. 2019. U.S. Food & Drug Administration. Available at: https://www.fda.gov/medical-devices/products-and-medical-procedures/implants-and-prosthetics
Implanted Medical Device. 2020. Science Direct. Available at: https://www.sciencedirect.com/topics/engineering/implanted-medical-device
Implantable Medical Devices. 2015. American Heart Association. Available at: https://www.heart.org/en/health-topics/heart-attack/treatment-of-a-heart-attack/implantable-medical-devices
Leading Medical Device Companies in 2020. 2021. University Lab Partners. Available at: https://www.universitylabpartners.org/blog/leading-medical-device-companies-2020
